How EU Regulations Affect Research Chemical Availability
How EU Regulations Affect Research Chemical Availability
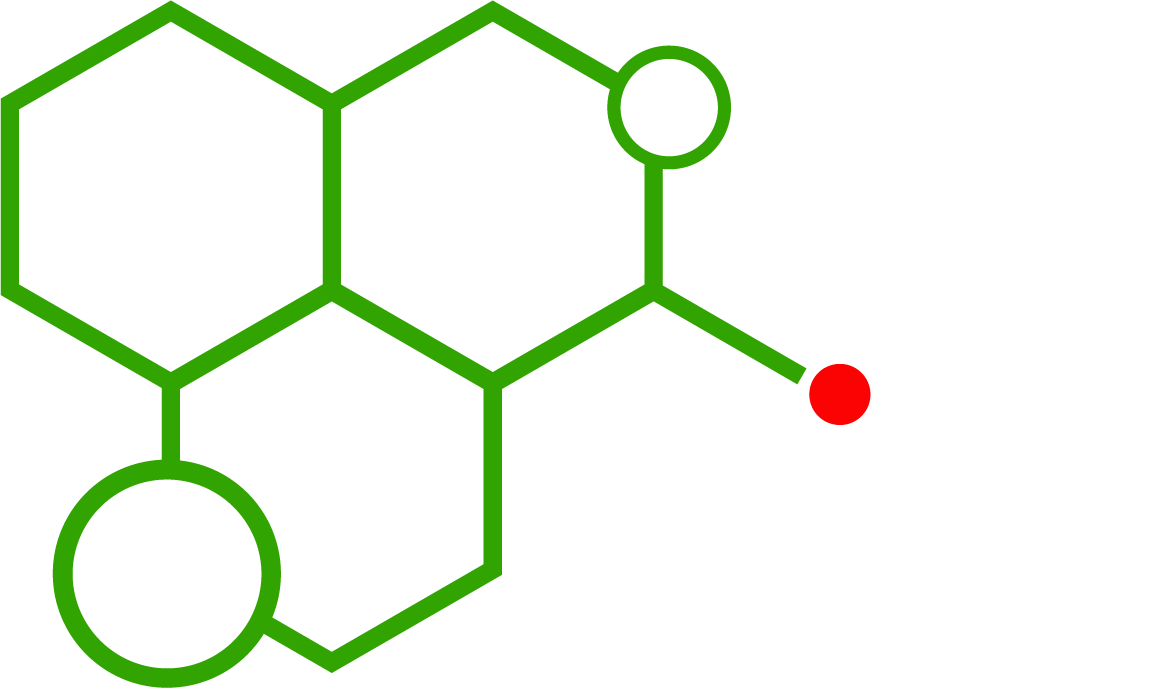
EU Research Chemical Regulations directly impact the accessibility of research chemicals across European laboratories. For scientists and institutions, understanding these regulations is essential to ensure legal compliance, safety, and smooth laboratory operations. Compounds such as 3-CMC, 5-MAPB, MDPHP, and JWH-210 are among the many substances affected by evolving European legal frameworks.
The EU Regulatory Framework for Research Chemicals
The European Union oversees the legal status of research chemicals through various bodies and directives:
- European Monitoring Centre for Drugs and Drug Addiction (EMCDDA): Identifies and evaluates emerging substances.
- Council of the European Union: Issues binding decisions to schedule and control substances across member states.
- National Authorities: Individual countries implement EU rules and may impose additional restrictions.
Official reports from the EMCDDA provide valuable insights into trends and controls affecting availability.
How Regulations Influence Availability
Regulations affect both the distribution and use of research chemicals:
- Controlled Access: Some substances like ADB-BUTINACA and 5Cl-ADB-A require permits or special licenses.
- Market Limitations: Availability can differ between EU member states due to local enforcement practices.
- Administrative Burden: Researchers must maintain detailed records and comply with SDS documentation.
Licensing and Compliance Requirements
To legally obtain and study regulated chemicals, laboratories must:
- Apply for national research licenses.
- Ensure secure storage following storage protocols.
- Use proper protective equipment during handling.
These measures ensure research integrity and compliance with EU standards.
Challenges Faced by Researchers
EU regulations create several challenges for laboratories, including:
- Supply Restrictions: Sudden scheduling of compounds like 2-MMC can disrupt research projects.
- Legal Complexity: Researchers must navigate both EU-level and national laws.
- Slower Innovation: Increased oversight may delay access to novel research compounds.
Despite these challenges, regulatory oversight aims to minimize public health risks while allowing controlled scientific progress.
Best Practices for Maintaining Access
To maintain access to research chemicals under EU law, laboratories should:
- Stay updated with EU legislation and national legal changes.
- Partner with trusted suppliers like Maxon Chemicals who provide compliant, research-grade substances.
- Maintain robust safety practices, from spill management to proper SDS documentation.
Conclusion
The influence of EU Research Chemical Regulations on availability cannot be overstated. From cannabinoids like JWH-210 to cathinones such as MDPHP, the ability to obtain and study these substances depends on compliance with EU frameworks. By staying informed, following licensing requirements, and prioritizing safety, laboratories can continue their work legally and responsibly.
Related resources:
 Cart is empty
Cart is empty 
Add a Comment